Information about the Company
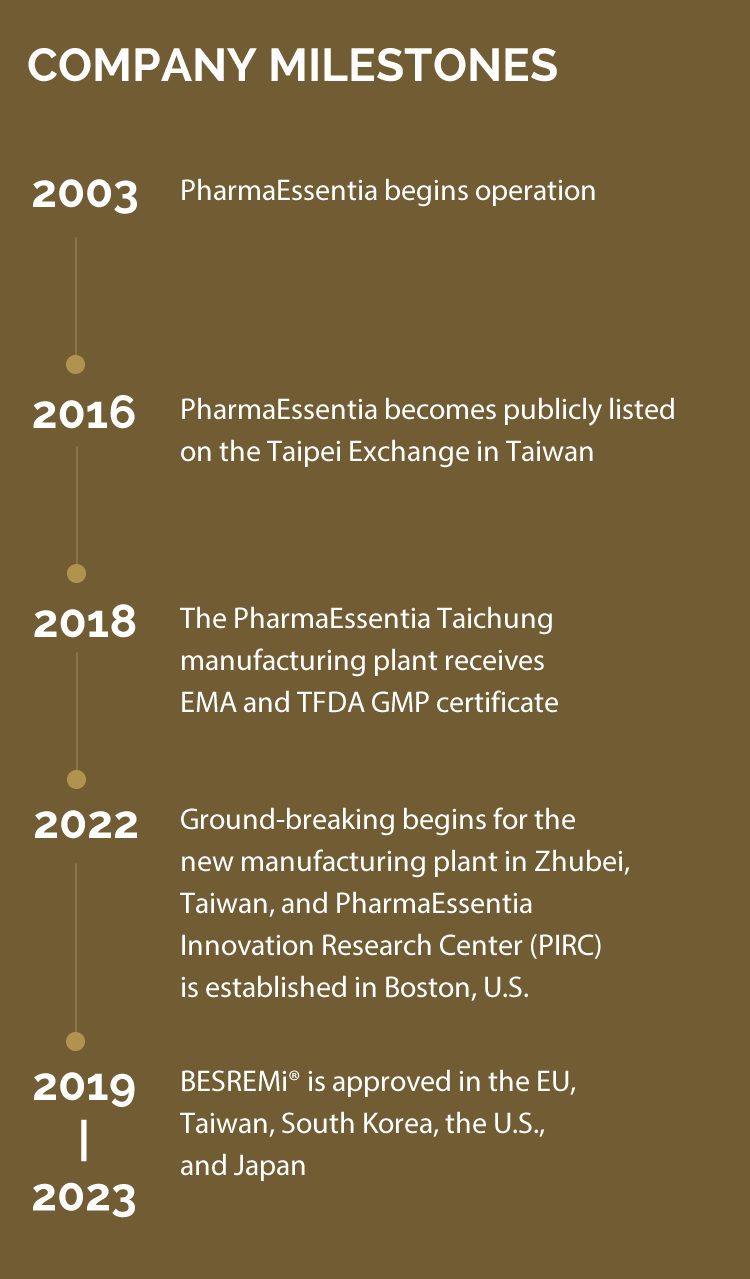
PharmaEssentia Corp. (PharmaEssentia) (TPEx: 6446), headquartered in Taipei, Taiwan, is a leading fully integrated biopharmaceutical company in Taiwan. Leveraging deep expertise and proven scientific principles, PharmaEssentia aims to deliver new biologics for challenging diseases in hematology and oncology, currently with one approved product and a diversifying pipeline. The company was founded in 2000 by a team of Taiwanese-American executives and renowned scientists from U.S. biotechnology and pharmaceutical companies. Today, PharmaEssentia is expanding its global presence with operations in the U.S., Japan, China, Korea, and Singapore, along with a world-class biologics production facility in Taichung, Taiwan. PharmaEssentia has fully integrated capabilities, including R&D and cGMP production facilities in Taiwan.
PharmaEssentia’s lead product, BESREMi® (Ropeginterferon alfa-2b, also known as P1101), is a novel, long-acting, mono-pegylated proline interferon with improved pharmacokinetic properties and demonstrated improved tolerability and convenience. To date, BESREMi® has been approved to treat adult patients with polycythemia vera (PV) and has been used by patients worldwide. PharmaEssentia is approaching its goal to address unmet medical needs, explore and develop new drugs and technologies, and provide patients with affordable and accessible drugs.
Corporate Culture & Company Activities
The company’s motto is “Better Science, Better Lives; One Team, One Mission”. Excellent employees are the cornerstone of sustainable business operations and the key to excellence. With hundreds of employees worldwide, PharmaEssentia adheres to a people-oriented philosophy by providing a comprehensive benefits system, diverse learning resources, and competitive salaries to enhance employee core competencies and corporate competitiveness. By attracting professional technology talents to form a high-quality workforce, PharmaEssentia creates a safe, healthy, and happy workplace environment for employees.
In 2022, PharmaEssentia’s sustainability report and the company itself received several awards. Continuously striving for excellence, PharmaEssentia has responded to SDG 13 - Climate Action by adopting the Task Force on Climate-related Financial Disclosures (TCFD) framework for the first time in 2022. The company completed a climate change risk and opportunity assessment, laying the foundation for climate governance and contributing its efforts to achieving global emission reduction targets. PharmaEssentia also sponsored projects for the Digital/ Tele-Medicine in Rural Area Program by the Digital Humanitarian Association, intending to help minority groups in rural areas receive timely medical care with digital assistance.
PharmaEssentia Corp. (PharmaEssentia) (TPEx: 6446), headquartered in Taipei, Taiwan, is a leading fully integrated biopharmaceutical company in Taiwan. Leveraging deep expertise and proven scientific principles, PharmaEssentia aims to deliver new biologics for challenging diseases in hematology and oncology, currently with one approved product and a diversifying pipeline. The company was founded in 2000 by a team of Taiwanese-American executives and renowned scientists from U.S. biotechnology and pharmaceutical companies. Today, PharmaEssentia is expanding its global presence with operations in the U.S., Japan, China, Korea, and Singapore, along with a world-class biologics production facility in Taichung, Taiwan. PharmaEssentia has fully integrated capabilities, including R&D and cGMP production facilities in Taiwan.
PharmaEssentia’s lead product, BESREMi® (Ropeginterferon alfa-2b, also known as P1101), is a novel, long-acting, mono-pegylated proline interferon with improved pharmacokinetic properties and demonstrated improved tolerability and convenience. To date, BESREMi® has been approved to treat adult patients with polycythemia vera (PV) and has been used by patients worldwide. PharmaEssentia is approaching its goal to address unmet medical needs, explore and develop new drugs and technologies, and provide patients with affordable and accessible drugs.
Corporate Culture & Company Activities
The company’s motto is “Better Science, Better Lives; One Team, One Mission”. Excellent employees are the cornerstone of sustainable business operations and the key to excellence. With hundreds of employees worldwide, PharmaEssentia adheres to a people-oriented philosophy by providing a comprehensive benefits system, diverse learning resources, and competitive salaries to enhance employee core competencies and corporate competitiveness. By attracting professional technology talents to form a high-quality workforce, PharmaEssentia creates a safe, healthy, and happy workplace environment for employees.
In 2022, PharmaEssentia’s sustainability report and the company itself received several awards. Continuously striving for excellence, PharmaEssentia has responded to SDG 13 - Climate Action by adopting the Task Force on Climate-related Financial Disclosures (TCFD) framework for the first time in 2022. The company completed a climate change risk and opportunity assessment, laying the foundation for climate governance and contributing its efforts to achieving global emission reduction targets. PharmaEssentia also sponsored projects for the Digital/ Tele-Medicine in Rural Area Program by the Digital Humanitarian Association, intending to help minority groups in rural areas receive timely medical care with digital assistance.



Better Science, Better Lives; One Team, One Mission.
Achievements and Impact
PharmaEssentia has over 500 employees worldwide, with a 30.9% growth rate for all employees in 2022. The average retention rate at the Taiwan headquarters is above 90%, with retention rates of over 80% for all levels of management and balanced gender ratios among employees. Since 2016, the number of female employees hired has exceeded that of male employees and is concentrated to among those under 30 years of age, providing ample employment opportunities for women while building a diverse workplace.
In addition to Taiwan, PharmaEssentia established operational bases in the U.S., China, Japan, South Korea, Singapore, and Hong Kong. The company recruited local high-level talents to accelerate market entry and establish operational service centers for local patients in each subsidiary and implemented efficient management to benefit patients.
PharmaEssentia has strong collaborations and support from the MPN community and medical experts. PharmaEssentia has not only actively participated in international MPN conferences but also co-organized MPN Asia, the annual international MPN symposium, to build a strong healthcare community in Asia. In 2022, PharmaEssentia also helped to establish the First MPN center in Taiwan with Chiayi Chang Gung Hospital to improve the research, development, and awareness of diseases and treatments.
Future Direction
PharmaEssentia is pursuing surging growth in four areas: further penetration of BESREMi® in key global markets; geographically expanding BESREMi® beyond the countries where it is currently approved; expanding additional indications for BESREMi® in MPNs and solid tumors; and advancing a pipeline of best-in-class/first-in-class therapies. The latter area includes new long-acting cytokines with PharmaEssentia’s innovative PEG platform, immunotherapy candidates, and innovative cell therapies.
PharmaEssentia is currently conducting a global phase 3 study for essential thrombocythemia (ET) as the second indication for Besremi® and preparing more collaboration with international cancer centers to explore possibilities of BESREMi®.
PharmaEssentia actively strives for innovation and drug development. In 2022, PharmaEssentia established an Innovation Research Center (PIRC) in the U.S. to enhance its global R&D capabilities, leverage world-leading Biotech talents and innovation resources in Boston, and accelerate discovery efforts across the related therapeutic fields of hematology, oncology, and immunology.
PharmaEssentia’s world-class cGMP biologics facility in Taichung is compliant with agencies’ requirements in Taiwan, the EU, the U.S., South Korea, and Japan. To meet the increasing demand for its product worldwide, PharmaEssentia is expanding a new manufacturing facility in Zhubei City, Taiwan.
PharmaEssentia has over 500 employees worldwide, with a 30.9% growth rate for all employees in 2022. The average retention rate at the Taiwan headquarters is above 90%, with retention rates of over 80% for all levels of management and balanced gender ratios among employees. Since 2016, the number of female employees hired has exceeded that of male employees and is concentrated to among those under 30 years of age, providing ample employment opportunities for women while building a diverse workplace.
In addition to Taiwan, PharmaEssentia established operational bases in the U.S., China, Japan, South Korea, Singapore, and Hong Kong. The company recruited local high-level talents to accelerate market entry and establish operational service centers for local patients in each subsidiary and implemented efficient management to benefit patients.
PharmaEssentia has strong collaborations and support from the MPN community and medical experts. PharmaEssentia has not only actively participated in international MPN conferences but also co-organized MPN Asia, the annual international MPN symposium, to build a strong healthcare community in Asia. In 2022, PharmaEssentia also helped to establish the First MPN center in Taiwan with Chiayi Chang Gung Hospital to improve the research, development, and awareness of diseases and treatments.
Future Direction
PharmaEssentia is pursuing surging growth in four areas: further penetration of BESREMi® in key global markets; geographically expanding BESREMi® beyond the countries where it is currently approved; expanding additional indications for BESREMi® in MPNs and solid tumors; and advancing a pipeline of best-in-class/first-in-class therapies. The latter area includes new long-acting cytokines with PharmaEssentia’s innovative PEG platform, immunotherapy candidates, and innovative cell therapies.
PharmaEssentia is currently conducting a global phase 3 study for essential thrombocythemia (ET) as the second indication for Besremi® and preparing more collaboration with international cancer centers to explore possibilities of BESREMi®.
PharmaEssentia actively strives for innovation and drug development. In 2022, PharmaEssentia established an Innovation Research Center (PIRC) in the U.S. to enhance its global R&D capabilities, leverage world-leading Biotech talents and innovation resources in Boston, and accelerate discovery efforts across the related therapeutic fields of hematology, oncology, and immunology.
PharmaEssentia’s world-class cGMP biologics facility in Taichung is compliant with agencies’ requirements in Taiwan, the EU, the U.S., South Korea, and Japan. To meet the increasing demand for its product worldwide, PharmaEssentia is expanding a new manufacturing facility in Zhubei City, Taiwan.
CORPORATE EXCELLENCE CATEGORY
PharmaEssentia Corp.
Information about the Company
PharmaEssentia Corp. (PharmaEssentia) (TPEx: 6446), headquartered in Taipei, Taiwan, is a leading fully integrated biopharmaceutical company in Taiwan. Leveraging deep expertise and proven scientific principles, PharmaEssentia aims to deliver new biologics for challenging diseases in hematology and oncology, currently with one approved product and a diversifying pipeline. The company was founded in 2000 by a team of Taiwanese-American executives and renowned scientists from U.S. biotechnology and pharmaceutical companies. Today, PharmaEssentia is expanding its global presence with operations in the U.S., Japan, China, Korea, and Singapore, along with a world-class biologics production facility in Taichung, Taiwan. PharmaEssentia has fully integrated capabilities, including R&D and cGMP production facilities in Taiwan.
PharmaEssentia’s lead product, BESREMi® (Ropeginterferon alfa-2b, also known as P1101), is a novel, long-acting, mono-pegylated proline interferon with improved pharmacokinetic properties and demonstrated improved tolerability and convenience. To date, BESREMi® has been approved to treat adult patients with polycythemia vera (PV) and has been used by patients worldwide. PharmaEssentia is approaching its goal to address unmet medical needs, explore and develop new drugs and technologies, and provide patients with affordable and accessible drugs.
Corporate Culture & Company Activities
The company’s motto is “Better Science, Better Lives; One Team, One Mission”. Excellent employees are the cornerstone of sustainable business operations and the key to excellence. With hundreds of employees worldwide, PharmaEssentia adheres to a people-oriented philosophy by providing a comprehensive benefits system, diverse learning resources, and competitive salaries to enhance employee core competencies and corporate competitiveness. By attracting professional technology talents to form a high-quality workforce, PharmaEssentia creates a safe, healthy, and happy workplace environment for employees.
In 2022, PharmaEssentia’s sustainability report and the company itself received several awards. Continuously striving for excellence, PharmaEssentia has responded to SDG 13 - Climate Action by adopting the Task Force on Climate-related Financial Disclosures (TCFD) framework for the first time in 2022. The company completed a climate change risk and opportunity assessment, laying the foundation for climate governance and contributing its efforts to achieving global emission reduction targets. PharmaEssentia also sponsored projects for the Digital/ Tele-Medicine in Rural Area Program by the Digital Humanitarian Association, intending to help minority groups in rural areas receive timely medical care with digital assistance.
PharmaEssentia Corp. (PharmaEssentia) (TPEx: 6446), headquartered in Taipei, Taiwan, is a leading fully integrated biopharmaceutical company in Taiwan. Leveraging deep expertise and proven scientific principles, PharmaEssentia aims to deliver new biologics for challenging diseases in hematology and oncology, currently with one approved product and a diversifying pipeline. The company was founded in 2000 by a team of Taiwanese-American executives and renowned scientists from U.S. biotechnology and pharmaceutical companies. Today, PharmaEssentia is expanding its global presence with operations in the U.S., Japan, China, Korea, and Singapore, along with a world-class biologics production facility in Taichung, Taiwan. PharmaEssentia has fully integrated capabilities, including R&D and cGMP production facilities in Taiwan.
PharmaEssentia’s lead product, BESREMi® (Ropeginterferon alfa-2b, also known as P1101), is a novel, long-acting, mono-pegylated proline interferon with improved pharmacokinetic properties and demonstrated improved tolerability and convenience. To date, BESREMi® has been approved to treat adult patients with polycythemia vera (PV) and has been used by patients worldwide. PharmaEssentia is approaching its goal to address unmet medical needs, explore and develop new drugs and technologies, and provide patients with affordable and accessible drugs.
Corporate Culture & Company Activities
The company’s motto is “Better Science, Better Lives; One Team, One Mission”. Excellent employees are the cornerstone of sustainable business operations and the key to excellence. With hundreds of employees worldwide, PharmaEssentia adheres to a people-oriented philosophy by providing a comprehensive benefits system, diverse learning resources, and competitive salaries to enhance employee core competencies and corporate competitiveness. By attracting professional technology talents to form a high-quality workforce, PharmaEssentia creates a safe, healthy, and happy workplace environment for employees.
In 2022, PharmaEssentia’s sustainability report and the company itself received several awards. Continuously striving for excellence, PharmaEssentia has responded to SDG 13 - Climate Action by adopting the Task Force on Climate-related Financial Disclosures (TCFD) framework for the first time in 2022. The company completed a climate change risk and opportunity assessment, laying the foundation for climate governance and contributing its efforts to achieving global emission reduction targets. PharmaEssentia also sponsored projects for the Digital/ Tele-Medicine in Rural Area Program by the Digital Humanitarian Association, intending to help minority groups in rural areas receive timely medical care with digital assistance.



Better Science, Better Lives; One Team, One Mission.
Achievements and Impact
PharmaEssentia has over 500 employees worldwide, with a 30.9% growth rate for all employees in 2022. The average retention rate at the Taiwan headquarters is above 90%, with retention rates of over 80% for all levels of management and balanced gender ratios among employees. Since 2016, the number of female employees hired has exceeded that of male employees and is concentrated to among those under 30 years of age, providing ample employment opportunities for women while building a diverse workplace.
In addition to Taiwan, PharmaEssentia established operational bases in the U.S., China, Japan, South Korea, Singapore, and Hong Kong. The company recruited local high-level talents to accelerate market entry and establish operational service centers for local patients in each subsidiary and implemented efficient management to benefit patients.
PharmaEssentia has strong collaborations and support from the MPN community and medical experts. PharmaEssentia has not only actively participated in international MPN conferences but also co-organized MPN Asia, the annual international MPN symposium, to build a strong healthcare community in Asia. In 2022, PharmaEssentia also helped to establish the First MPN center in Taiwan with Chiayi Chang Gung Hospital to improve the research, development, and awareness of diseases and treatments.
Future Direction
PharmaEssentia is pursuing surging growth in four areas: further penetration of BESREMi® in key global markets; geographically expanding BESREMi® beyond the countries where it is currently approved; expanding additional indications for BESREMi® in MPNs and solid tumors; and advancing a pipeline of best-in-class/first-in-class therapies. The latter area includes new long-acting cytokines with PharmaEssentia’s innovative PEG platform, immunotherapy candidates, and innovative cell therapies.
PharmaEssentia is currently conducting a global phase 3 study for essential thrombocythemia (ET) as the second indication for Besremi® and preparing more collaboration with international cancer centers to explore possibilities of BESREMi®.
PharmaEssentia actively strives for innovation and drug development. In 2022, PharmaEssentia established an Innovation Research Center (PIRC) in the U.S. to enhance its global R&D capabilities, leverage world-leading Biotech talents and innovation resources in Boston, and accelerate discovery efforts across the related therapeutic fields of hematology, oncology, and immunology.
PharmaEssentia’s world-class cGMP biologics facility in Taichung is compliant with agencies’ requirements in Taiwan, the EU, the U.S., South Korea, and Japan. To meet the increasing demand for its product worldwide, PharmaEssentia is expanding a new manufacturing facility in Zhubei City, Taiwan.
PharmaEssentia has over 500 employees worldwide, with a 30.9% growth rate for all employees in 2022. The average retention rate at the Taiwan headquarters is above 90%, with retention rates of over 80% for all levels of management and balanced gender ratios among employees. Since 2016, the number of female employees hired has exceeded that of male employees and is concentrated to among those under 30 years of age, providing ample employment opportunities for women while building a diverse workplace.
In addition to Taiwan, PharmaEssentia established operational bases in the U.S., China, Japan, South Korea, Singapore, and Hong Kong. The company recruited local high-level talents to accelerate market entry and establish operational service centers for local patients in each subsidiary and implemented efficient management to benefit patients.
PharmaEssentia has strong collaborations and support from the MPN community and medical experts. PharmaEssentia has not only actively participated in international MPN conferences but also co-organized MPN Asia, the annual international MPN symposium, to build a strong healthcare community in Asia. In 2022, PharmaEssentia also helped to establish the First MPN center in Taiwan with Chiayi Chang Gung Hospital to improve the research, development, and awareness of diseases and treatments.
Future Direction
PharmaEssentia is pursuing surging growth in four areas: further penetration of BESREMi® in key global markets; geographically expanding BESREMi® beyond the countries where it is currently approved; expanding additional indications for BESREMi® in MPNs and solid tumors; and advancing a pipeline of best-in-class/first-in-class therapies. The latter area includes new long-acting cytokines with PharmaEssentia’s innovative PEG platform, immunotherapy candidates, and innovative cell therapies.
PharmaEssentia is currently conducting a global phase 3 study for essential thrombocythemia (ET) as the second indication for Besremi® and preparing more collaboration with international cancer centers to explore possibilities of BESREMi®.
PharmaEssentia actively strives for innovation and drug development. In 2022, PharmaEssentia established an Innovation Research Center (PIRC) in the U.S. to enhance its global R&D capabilities, leverage world-leading Biotech talents and innovation resources in Boston, and accelerate discovery efforts across the related therapeutic fields of hematology, oncology, and immunology.
PharmaEssentia’s world-class cGMP biologics facility in Taichung is compliant with agencies’ requirements in Taiwan, the EU, the U.S., South Korea, and Japan. To meet the increasing demand for its product worldwide, PharmaEssentia is expanding a new manufacturing facility in Zhubei City, Taiwan.